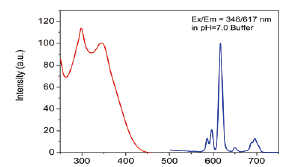
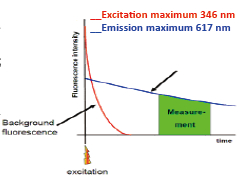
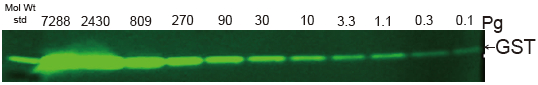
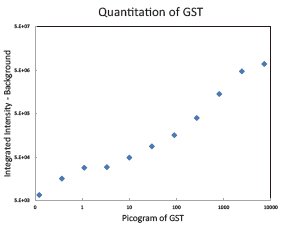
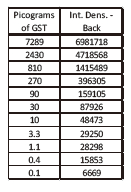
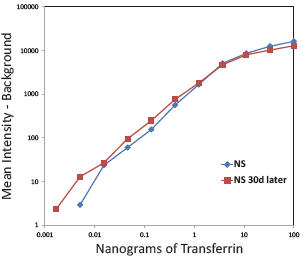
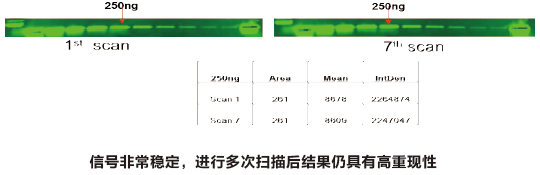
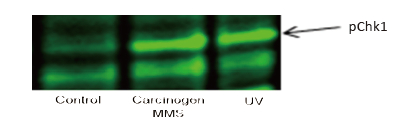
Western Blot protein detection and quantification based on time-resolved fluorescent labeling on a plate reader Vanitha Thulasiraman1, Jia-Ren Lin2, Cathy Olsen1, Rainer Muehlbacher3, Brian Quast1, Summary Protein detection is a very important project in the pharmaceutical and clinical research fields, and Western Blot (WB) testing is the most common of many protein detection methods. At present, there are many techniques for detecting proteins transferred onto WB membranes, including fluorescent labeling, silver staining, and chemiluminescence. However, each technology has its limitations, and people have been working hard to find technical methods to improve the quantitative accuracy and dynamic detection range of WBs. Here, we introduce a new protein analysis method for detecting WBs membranes on SpectraMax i3 or Paradigm multi-function microplate readers. The membrane is coated with a ruthenium-chelate or streptomycin-labeled secondary antibody to specifically bind to the protein under investigation. The ruthenium element label has a long fluorescence lifetime, up to the 1ms level, so fluorescence detection uses a time-resolved pattern that reduces the background of stray light from autofluorescence and other short life cycles. The WBs membrane was placed in a microplate reader and then an optimized scan of the WBs membrane was performed using a TRF cartridge. This method does not involve the detection of enzymes, and the ruthenium-chelate label has a strong photobleaching resistance, so the signal is very stable for weeks to months, which allows repeated scanning of the membrane and possible Comparison of the strength of the different strips and more precise quantification according to the standard. The TRF detection method uses a single photon counting mode, so the theoretical dynamic detection range is >105. In practical applications, the dynamic detection range is limited by the fluorescence saturation of high-content bands and the background factors caused by non-specific binding. Since there is no blooming phenomenon without using a CCD, which is a common problem in chemiluminescence and ordinary fluorescence detection, this method can provide very sharp strips and excellent image quality. The Sc anlater Western Blot detection system on this novel multi-function microplate reader detection platform provides excellent WBs detection performance with easy operation, sensitive detection and excellent signal stability. The instrument brings another major breakthrough in laboratory applications! principle The ScanLater Western Blot detection system simplifies the process of Western Blot, which uses a ruthenium-chelate label and scans on a microplate reader. No substrate is required, and the membrane is washed and directly scanned. The erbium element has a long fluorescence lifetime, up to 1ms, and the detection mode uses time-resolved mode (TRF), which greatly reduces autofluorescence and the rest of short-lived background stray light. Figure 1. Top left: Schematic diagram of protein detection. The secondary antibody is directly labeled with a ruthenium element. Upper right: 铕 element-chelate excitation and emission spectra. Bottom right: TRF detection mode, the fluorescence period of the yttrium element is compared with the decay period of the background fluorescence, and the detection of the signal takes a certain time delay to remove the background fluorescence interference. Materials and Methods material method Sensitivity and dynamic detection range Sensitivity and dynamic detection range testing was performed using GST, GST was diluted in 3X with 1X loading solution, then applied to 4-20% gradient gel for 30 min, then transferred to Immobilon FL membrane using biotinylated rabbits. Anti-GST labeling was carried out for 2 hours, followed by incubation with Eu-labeled streptomycin for 1 hour, then the membrane was washed and air-dried using a SpectraMax Paradigm multi-plate reader. The system exhibits a sub-pick-level detection sensitivity and a dynamic detection range of >4 logs. Figure 2. Above: GST gradient dilution image of SpectraMax Paradigm scan (Note: pseudo color scale is used to display large dynamic detection range); Below: total fluorescence intensity for each strip, statistics show the entire dynamic detection The range is 4logs, the linear detection range is 3logs, and image analysis uses Image J. Signal stability One of the most prominent advantages of strontium-labeling is the stability of the signal and its resistance to photobleaching, so that the stability of the western film-printed strip can be as long as several months. To show this, we performed a three-fold dilution of transferrin on a gel (4-20% gradient gel) for 30 min, then transferred onto Immobilon FL membrane, and incubated with rabbit anti-transferrin overnight at 4 °C, then with The sputum-labeled anti-rabbit IgG was incubated for 1 hour at 4 ° C, then the membrane was washed, air-dried, and scanned using the SpectraMax Paradigm on the day and one month later. • There is almost no loss of signal after 30 days • Highly quantifiable results In addition, the strip can be repeatedly scanned and the signal is not reduced. The 2-fold gradient of transferrin is run (4-20% gradient gel) for 30 min, then transferred onto Immobilon FL membrane, and rabbit anti-- Transferrin was incubated for 2 hours and then incubated with strontium-labeled anti-rabbit IgG for 4 hours at 4 ° C, then washed, air-dried, and scanned multiple times using SpectraMax Paradigm. application Figure 5. HEK293 cells were treated with different concentrations (0, 50, 100 ppm) of carcinogen MMS (an alkylating agent) and then tested and compared using the Scanlater TRF and chemiluminescence methods (Stanford University). • Detection of trace amounts of endogenous phosphorylated pChk1 (involved in UV & MMS-damaged DNA repair processes) using ScanLater Western Blot technology. Figure 6. Detection of phosphorylated pChk1 (Stanford University) in HEK-293 cells treated with carcinogen MMS and UV radiation, respectively. • Detection of trace amounts of endogenous phosphorylated JNK1 protein produced during cell stimulation and inhibition by signal transduction using ScanLater Western Blot; Figure 7. Detection of phosphorylated JNK1 protein in natural human lung fibroblasts (NHFL) after stimulation and inhibition (results from trial clients). to sum up Flood Tube,Flood Barrier Tubes,Plastic Poly Tube Flood Barrier,Tube Wall Denilco Environmental technology(Suzhou)Co., Ltd. , https://www.wflood.com
Michael Katzlinger3, Josef Atzler3, Karlene A. Cimprich2, Evan F. Cromwell1 1Molecular Devices LLC, Sunnyvale, California, USA; 2Department of Chemical & Systems Biology, Stanford University, Stanford, California, USA; 3Molecular Devices GmbH, Salzburg, Austria 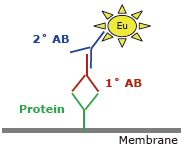
• Scanlater Western Blot Kit (10X Washing Buffer, 5X Blocking Buffer, Eu-Labeled Anti-Mouse IgG, Eu-Labeled Anti-Rabbit IgG, Eu-Labeled Streptavidin), supplied by Molecular Devices, LLC.
• Western Blot membranes were scanned using a SpectraMax Paradigm or SpectraMax i3 multi-function reader (Molecular Devices).
• Glutathione S-transferase (GST), biotinylated rabbit anti-GST, mouse anti-GST, rabbit anti-transferrin, transferrin (Abcam). Chemiluminescent substrate and HRP-secondary antibody (Millipore).
• TGX 4-20% gel, double-labeled protein standard, running buffer, loading buffer, transfer buffer, protein transfer system (Bio-Rad Laboratories), Immobilon FL membrane (Millipore).
• At 200V, the protein was run in TGX 4-20% for 30min, then transferred to PVDF membrane by Trans Blot Turbo for 7min, then rinsed with TBST for 30s, and then blocked with 1X blocking solution for 1h.
• The primary antibody is added directly to the blocking solution at a certain dilution ratio, placed at room temperature for 2 hours or 4 ° C overnight, and washed with a 1X wash solution three times for 5 minutes each time.
• The secondary antibody was diluted 1:5000 with 1X blocking solution, then incubated on the membrane for 60 min, then washed 1X for 5 min each time, then rinsed in deionized water for 15 s, dried and ready for scanning.
• Scan detection on SpectraMax Paradigm or SpectraMax i3 multi-function readers, using SoftMax® Pro software for analysis. 

• Detection of tracely expressed endogenous ubiquitinated Rad-18 protein (involved in UV-damaged DNA repair processes) using Scanlater Western Blot technology. 
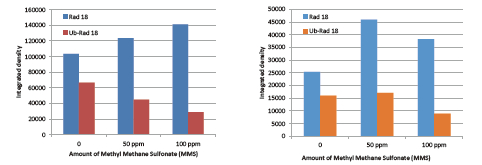

• Sensitivity: Ability to detect endogenous phosphorylated and ubiquitinated proteins in ultra-low levels of human cells;
• Background Elimination: 铕 element time-resolved fluorescent labeling with ultra-long fluorescence lifetime up to 1ms, using TRF detection mode can greatly reduce auto-fluorescence and background noise from other sources;
• Save time: no ECL-like time-consuming optimization process is required;
• Reduced costs: no need for costly X-ray film and developer;
• Plug and Play: High-performance WB inspection on the SpectraMax i3 or SpectraMax Paradigm multi-function microplate readers at any time greatly expands its use in laboratory research.