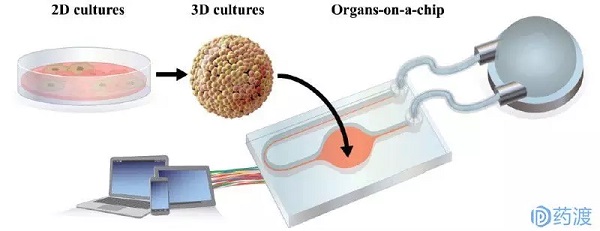
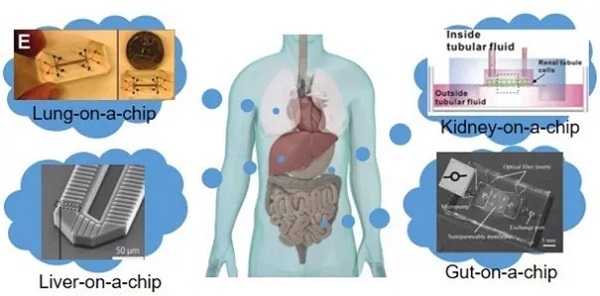
Release date: 2018-04-02 The high failure rate of new drug research and development is well known. Take anticancer drugs as an example. The annual cost of research and development failures in the United States is between $800 million and $1.4 billion. On the one hand, relying on animal experiments to obtain preclinical data to predict drug efficacy, toxicity, and PK (pharmacokinetic) parameters in humans is a "standard operation" in the development of new drugs. However, due to the differences between species of animals and humans, the prediction bias is inevitable, leading to the failure of bridging of many preclinical and clinical trials, which ultimately leads to development failure. On the other hand, in vitro cell models are used to replace animal experiments. The current in vitro models rely mainly on two-dimensional or simple three-dimensional cell culture systems. Simple and fast high-throughput is its advantage and is widely used in the screening of early compounds. However, the simple cell model cultured by the culture dish and the multi-well plate is incomplete in physiological function, and does not consider the interaction between tissues, and does not accurately reflect the mechanism under physiological conditions, which is misleading for the selection of compounds. Therefore, it is difficult to accurately predict the behavior of drugs in humans, whether in animal or in vitro experiments. The industry urgently needs a drug screening platform that is more relevant to physiological functions and has higher precision. In the 1990s, scientists who studied Micro Total Analysis Systems (μTAS) proposed the concept of organ-on-a-chip based on microfluidic technology. As a potential new tool for cell testing, at the beginning of organ chip research, the concept was mainly put to the fore, and it was far from the practical application goal. In recent years, with the maturity of technology, more and more funds have been invested in this field, and the application prospects are clear. This article has organized the current state of technology development of organ chips and how to participate in the problems encountered in drug development and application. Microfluidics and cell-inspired inspiration Microfluidic technology integrates micro/nano technology, chemical sensors and analytical chemistry. Microfluidic devices are typically fabricated by semiconductor microfabrication techniques such as lithography or soft-printing, and can be used for chemical analysis of microchannels or microstructures, also known as "lab-on-a" Chip). As described above, in vitro cell evaluation systems have deficiencies such as cell inactivation and loss of physiological function, even in primary cultured cells. At the same time, the cell culture environment of the classical method is relatively static, and the drug to be tested mainly enters the cell through passive diffusion, which is quite different from the real situation in the body. In vivo cells can obtain oxygen and nutrients through the blood, as well as chemical and physiological stimuli, such as cell stretching and shear stress caused by the microenvironment. These in vitro and in vivo differences in morphology and environment have led to the loss of function of the cultured cell lines. In order to fill this huge gap, since 2000, μTAS and tissue engineering researchers have jointly developed cell devices based on microfluidic technology. It has the advantages of liquid resonance, cell adhesion, mechanical stimulation and other functions to replicate tissue function, forming the prototype of the current organ chip. In recent years, the development of micro-manufacturing, cell engineering, and imaging technology has helped organ chips become innovative technologies for in vitro bionics, attracting financial support from governments and institutions such as NIH, FDA, Europe, and Japan. Especially with the development of induced pluripotent stem cell (iPS) technology, organ chips have been widely used in new drug development as a tissue model or disease model. Organ chip application Currently available organ chips include the lungs, liver, kidneys, and intestines, which are described below. Chip lung The earliest development of chip lungs in organ chips, this microfluidic cell device is also known as "breathing lungs." The chip lungs invented by the Ingber research team at Harvard University consist primarily of a two-layer channel structure that is vertically separated by a microporous membrane of silicone and polydimethylsiloxane. The upper layer of the membrane is alveolar epithelial cells, and the lower layer is vascular endothelial cells, which are administered with flowing air and medium, respectively. In addition to the function of replicating lung cells, this microporous membrane can also undergo periodic contraction under the action of internal pressure changes on both sides of the channel, simulating the physiological diastolic and contractile movements of the alveoli during respiration. Using this chip lung, the researchers found that vascular endothelial cells can cause inflammatory stress and high expression of ICAM-1 in the environment exposed to TNF-α and bacteria. In another toxicity evaluation, the microparticles were able to get more into the blood vessel side under the stretched state, which is consistent with observations in animal experiments. All of the above studies have shown that chip lung as an in vitro disease model can meet the needs of new drug development. Chip liver The important role played by the liver in drug metabolism is not to be described. It is very important to accurately predict the liver's ability to metabolize drugs and the toxic accumulation of drugs in the liver during the development of new drugs. There is a lack of enzyme activity and function of hepatocytes used in current in vitro experiments. The Powers research team used microfluidic technology to construct a three-dimensional morphological structure of the liver of the chip, which was placed under continuous perfusion. Each channel of this device consists of a filter with retained cell function and a cell culture chamber, which is perfused from top to bottom. The flow rate is set based on the estimated oxygen consumption of the cells to provide a fluid shear stress that conforms to the physiological range. Studies have shown that this chip liver can accumulate cells to form a hepatic acinar structure and maintain its structure and corresponding physiological functions within two weeks. In addition to the structure to be as biosimilar as possible, it is also important to maintain the polarity of liver cells. Centered on the central vein, hepatocytes are arranged radially into hepatic cell cords, and there are bile ducts between adjacent hepatocytes. Bile is collected through the bile ducts and finally excreted through the bile ducts. There are many drug metabolites in the bile that are biotransformed in the liver and are excreted through the bile duct. Therefore, bile excretion is an important part of in vitro studies of drug metabolism. Nakao and his team invented an in vitro hepatic lobular model device that replicates the function of hepatic cell cords. The cell culture zone of this device arranges the hepatocytes in two rows in parallel to form a structure similar to that of hepatocytes. Studies have found that these neatly arranged cells can grow along the cell line to form a bile duct structure. The researchers used the drug CDFDA to test the metabolic function of the chip. CDFDA was able to metabolize to a fluorescent product, CDF, by hepatic esterase, and then excreted through the bile. After the CDFDA-containing medium was passed through the vascular channel of the device, CDF was detected in the "biliary-tube" structure formed by the cells. This result demonstrates that the liver of the chip fabricated by microfluidic technology can simulate tissue morphology and corresponding physiological functions. Various organ chips based on microfluidic technology (picture from reference article) Chip kidney The kidney is responsible for drug metabolism and excretion in the body. In the preclinical screening phase, evaluation of kidney accumulation and toxicity of candidate compounds is usually done in vivo in animals, and there is no better in vitro model. There are also a few drug failures caused by the differences in nephrotoxicity of the species. The development of a highly effective and accurate in vitro nephrotoxicity evaluation system is urgently needed for the development of new drugs. Furthermore, if an in vitro model can not only predict nephrotoxicity, but also reflect the mechanism behind kidney disease, it is better to use for screening related drugs. The current chip kidney is to lay the MDCK cell line and the human HK-2 cell line on the bottom of the microchannel of the chip, and then apply the corresponding shear stress. Studies have shown that this cell structure can gradually increase in thickness, increase in Na/K ATPase expression and cilia formation, and basically achieve the purpose of simulating the physiological function of kidney cells. Another microporous membrane chip model that mimics renal tubular reabsorption is to create physiological stresses on the apical side including changes in salt concentration and osmotic pressure by adding hormones such as vasopressin and aldosterone to the basal side channels. The researchers found that shear stress not only regulates cell growth, but also promotes Pgp protein expression, cilia growth, and cellular albumin glucose uptake. The Musah team replicated the glomerular structure using microfluidic technology and human iPS-derived podocytes. It can mimic physiological functions including shear stress and can be used as an in vitro kidney model to screen cancer drugs. The above results show that even if the structure and function are as complex as the kidney, an in vitro model that maintains basic kidney function can be obtained by using microfluidic chip technology. Chip intestine As the main organ responsible for digestion and absorption, the intestine, especially the small intestine, acts as a barrier to oral drugs, and predicting its absorption function is important for the development of new drugs. Kimura's research team invented a small intestine chip device with a light detection system. A separate cavity separated by a microporous membrane that cultures Caco-2 cells on two membranes, the intestinal microarray has a transporter function, and can evaluate drug absorption, but the physiological function is incomplete. Kim's research team invented the intestine of the chip to simulate physiological stresses such as shear stress and periodic stretching. Caco-2 cells exposed to this biomimetic environment were able to grow villi and differentiate cells. In order to replicate the physiological environment of the complex, the researchers also incubated the intestinal cells of this system with many commensal bacteria. The improved chip intestine can achieve the same results as previous animal experiments or human experiments, indicating that the intestine of the chip can serve as an in vitro platform for evaluation of intestinal physiological functions and disease mechanisms. "body" chip The use of microfluidic technology to prepare organ chips is one thing. It is another matter to correlate different organ chips to form a more complex "body" chip, because it is very important to accurately predict the interaction between tissues and organs. difficult. Similar devices have been tried to obtain continuous PK and ADME (absorption, distribution, metabolism, and excretion) data for drugs, and to model these parameters to predict the metabolic process of drugs in the human body. Shuler and his colleagues are engaged in cutting-edge research in the field of bioengineering. They have invented a body chip that includes multiple tissue chambers and co-cultures different tissue cells, called uCCA (micro cell culture analog). The researchers used the chip system to compare the interactions between the anti-tumor drug Tegafur and the tissue and organ in the two routes of PO and IV. The results showed that the system can obtain similar data similar to animal experiments. Imura's research team has also created another body chip that integrates the absorption function of small intestinal cells, the metabolic function of liver cells, and the excretion of kidney cells. The chip was used to compare a series of anti-tumor drugs with different absorption levels and therapeutic mechanisms, and the difference in drug efficacy proved the feasibility of the device. Although the above chip device simulates the interrelationship between organs, the obtained parameters do not directly reflect physiological functions. Kimura's research team's body chip incorporates various physiological parameters in the body, such as tissue volume ratio and blood flow, which can be used for anti-tumor drug efficacy evaluation. "Body" chip based on PBPK principle (picture from reference article) It must be admitted that these body chip technologies are far from mature, and they cannot replicate all the physiological functions in the body. In fact, the importance of the body chip is not to create a complex "micro-human body", but to use in vitro technology to reveal the interactions between drugs and metabolic pathways in the body, and to use the obtained parameters to predict drug prediction. In vivo process. In other words, the body chip needs to be used in conjunction with the PK model to predict and explain mechanisms and phenomena. The body chip described above can be applied to different pharmacological toxicity studies, by introducing different cells and matrices to obtain data, and then combining mathematical models to construct a PK prediction evaluation system. The challenges facing organ chip development Despite rapid development, organ chips are not a substitute for animal experiments, and they have their own bottlenecks in both chip technology and biological mechanism. The first is the short board of organ chip detection technology. The current evaluation system is nothing more than direct observation or measurement of functional parameters. But the most useful in vitro model should be to have a system for observing and recording specific physiological responses to different biostimulatory signals. Therefore, we need to continue to develop detection technology and biochemical technology, so that biochemical analysis can be better completed in the micro-fluidic device's miniature space. Secondly, the complicated microfluidic chip preparation process also limits the development of this technology. Only by implementing automated preparation, organ chips can be truly promoted. In addition, there is a biological problem that is the source of cell lines. The immortalized cell lines currently used are mostly tumor cells derived from the loss of tissue function; although the use of primary human cells can relatively accurately predict PK parameters, there are also donations. Source and cost issues. Perhaps the widespread use of human iPS-induced cells in the future can make up for this deficiency. Although there are no examples of successful application of organ chips in drug development, organ chips are undoubtedly a promising drug development aid. The development of cross-technology often faces the same problem, which is interdisciplinary technical communication. Just like the development of organ chips, biologists know where the problem is, but don't know how to solve it; engineers know how to make chips, but they don't know what problems it can solve. Only by integrating technologies in the fields of medicine, pharmacy, biology and engineering can we make organ chips more powerful and better for human health. Reference article: The Homunculus Metaphor Revisited In DrugDiscovery: Body-on-a-Chip. Organs-on-a-chip: A new paradigm for toxicological assessment and preclinical drug development. The Homunculus Metaphor Revisited In DrugDiscovery: Body-on-a-Chip Source: Yaodu Industrial Laser Distance Sensor
Industrial
Laser Distance Sensor, we also call it secondary development laser distance
module, which support TTL level and CMOS. The laser range sensor can be widely
used in professional surveying, mapping, construction, robots, hunting arrows,
industrial monitoring and automated measurement applications in electricity,
transportation, etc. Our laser distance module supports data communication with
RS232, USB with a simple adapter. The results of laser distance sensor can be
evaluated with Arduino. We are always looking ahead, hoping we can make every
measurement simple in life!
Parameters
of M703A:
Accuracy
±1
mm (0.04 inch)
Measuring
Unit
mm
Measuring
Range (without Reflection)
0.03-60m(150m can customize)
Measuring
Time
0.125~3
seconds
Laser
Class
Class
II
Laser
Type
620-690nm,
<1mW
Size
45*25*12mm (±1 mm)
Weight
About 10g
Voltage
DC2.0~3.3V
Electrical
Level
TTL/CMOS
Frequency
8Hz(20Hz can customize)
Operating
Temperature
0-40 ℃ (32-104 ℉ )
Storage
Temperature
-25~60 ℃ (-13~140 ℉)
Laser Distance RS232,Arduino Distance Module,Laser Module RS232 Chengdu JRT Meter Technology Co., Ltd , https://www.irdistancesensor.com