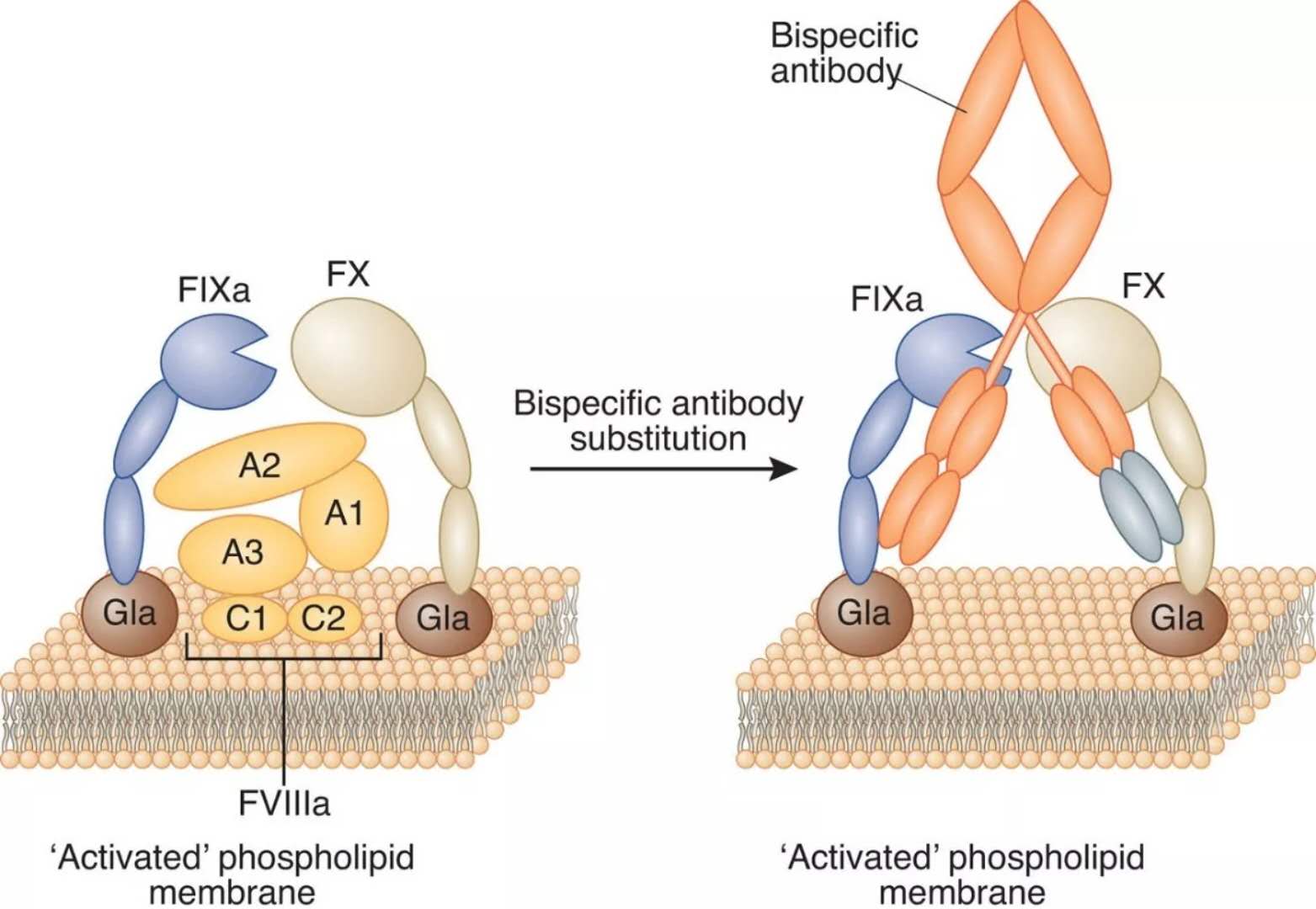
Roche's new hemophilia drug announced the latest data: all up to standard! May 22, 2018 Source: WuXi PharmaTech Recently, Genentech, a member of the Roche Group, announced data on two key Phase 3 studies (HAVEN 3 and HAVEN 4). The HAVEN 3 study evaluated the efficacy of Hemlibra (emicizumab-kxwh) prophylaxis in weekly or biweekly patients with hemophilia A who did not have a Factor VIII inhibitor. The HAVEN 4 study evaluated the efficacy of Hemlibra administered every four weeks in patients with hemophilia A with or without factor VIII inhibitors. Data from these two studies were presented at the World Congress of Hemophilia (WFH) World Congress in 2018. Hemophilia A is a serious genetic disease in which the patient's blood cannot coagulate normally, resulting in uncontrolled spontaneous bleeding. Hemophilia A affects approximately 320,000 people worldwide, and 50-60% of them have a serious form of the disease. Patients with hemophilia A lack a coagulation protein called factor VIII. In healthy people, Factor VIII brings together Factor IXa and Factor X when bleeding occurs, a key step in coagulation that can help stop bleeding. Depending on the severity of the condition, people with hemophilia A may bleed frequently, especially in joints or muscles, causing severe health problems such as pain, chronic swelling, deformity, mobility problems, and long-term joint damage. A serious complication of hemophilia A is that the patient develops an inhibitor of factor VIII replacement therapy, an antibody developed by the body's immune system that binds and blocks the replacement factor VIII, making it inadequate. Control the level of bleeding. Most patients with hemophilia A develop a factor VIII inhibitor and intermittent or prophylactic infusion bypass (BPA) therapy to control bleeding. This patient population also has huge medical needs that are not being met. Hemlibra is a bispecific factor IXa and a factor X directed antibody. It aggregates the proteins needed to activate the natural coagulation cascade, Factor IXa and Factor X, to restore the clotting process in patients with hemophilia A. Hemlibra is a prophylactic treatment that can be administered by subcutaneous injection of a ready-to-use solution once a week. The drug was approved by the US FDA in November last year for routine prevention or reduction of bleeding events in adults and children with hemophilia A with factor VIII inhibitors. ▲The mechanism of action of bispecific factor IXa and factor X directed antibodies (Source: Nature) In the HAVEN 3 study conducted this time, patients who were 12 years of age or older without factor VIII inhibitors who received Hemlibra prophylaxis every week or every week had a 96% reduction in bleeding compared to patients who did not receive prophylactic treatment (p <0.0001) and 97% (p<0.0001). In addition, 55.6% (95% CI: 38.1-72.1) and 60% (95% CI: 42.1-76.1) in the treatment group experienced zero bleeding per week or two weeks, compared with 0% in the control group ( 95% CI: 0.0-18.5). Importantly, in a patient-to-patient comparison, weekly Hemlibra prophylaxis showed a superior response compared with previous standard treatment in patients who underwent a prospective non-invasive study (NIS). Bleeding was reduced by 68% (p < 0.0001). In addition, 93.7% (n=89/95, 95% CI: 86.8-97.7) of all patients who completed the treatment preference survey preferred Hemlibra treatment, while 97.8% (n=45/46) preferred Hemlibra in the patient's internal comparison. treatment. No unexpected or serious adverse events (AEs) associated with Hemlibra were found, the most common AE being consistent with previous studies. In the one-arm HAVEN 4 study, the median annual bleeding rate (ABR) was 0.0 (IQR: 0.0-2.1) for patients 12 years of age or older who received Hemlibra prophylaxis with or without Factor VIII inhibitors, including 56.1. People with % (95% CI: 39.7-71.5) experienced zero bleeding, and 90.2% (95% CI: 76.9-97.3) experienced three or fewer bleedings. These results indicate that every four weeks of Hemlibra administration can provide meaningful clinical control for bleeding in patients with hemophilia A with or without factor VIII inhibitors. In addition, all participants who completed the patient preference survey (n=41/41, 95% CI: 91.4-100.0) preferred Hemlibra treatment. No serious AE associated with Hemlibra was found, the most common AE being consistent with previous studies. "As demonstrated by the significantly reduced bleeding in the comparison of patients in the HAVEN 3 study, Hemlibra was the first drug to show superiority to the previous standard therapy, Factor VIII prophylaxis," University of the Witwatersrand, South Africa. Dr. Johnny Mahlangu, a professor of health sciences, said: "Even with current preventive treatments, many people with hemophilia A will still bleed and cause long-term joint damage, and they need more treatment options." "These new key data show that Hemlibra can control bleeding in patients with hemophilia A, while providing less flexibility in subcutaneous drug delivery options," said Dr. Sandra Horning, chief medical officer and head of global product development at Genentech. “With these data, we now have positive results from all four Phase 3 clinical trials that enhance the overall efficacy and safety of Hemlibra and help improve the care of all patients with hemophilia A. †According to data from the HAVEN 3 study, the US FDA issued a breakthrough therapy award for Hemlibra last month for patients with hemophilia A who did not have a factor VIII inhibitor. We expect these new results to accelerate the application of this drug to a wider range of hemophiliacs. Reference materials: [1] Genentech's HEMLIBRA (emicizumab-kxwh) Reduced Treated Bleeds by 96 Percent Compared to No Prophylaxis in Phase III Haven 3 Study in Hemophilia A Without Factor VIII Inhibitors [2] Genentech's Hemlibra Significantly Reduces Bleeding in Late-Stage Hemophilia Studies [3] WuXi PharmaTech - Heavy! The first new hemophilia drug was approved today in 20 years
The kelp soup is very convenient to eat .
Select the tender parts of the kelp, the delicious soup comes from the deep sea. After opening the paper cups, it can be soaked in boiling water for a minute, which is delicious and nutritious. Rich in nutrients needed by the human body,It can be all kinds of people, especially teenagers and young women,.It's good for health and beauty.
Kelp Soup,Kelp Broth,Wakame Seaweed Soup,Vegetarian Seaweed Soup Shandong Haizhibao Ocean Science and Technology Co.,Ltd. , https://www.haizhibaoseafood.com