Small Molecule Peptide,Collagen Peptide,Yam Peptide Powder,Herb Ginseng Peptide Fufeng Sinuote Biotechnology Co.,Ltd. , https://www.ffsinuoteplant.com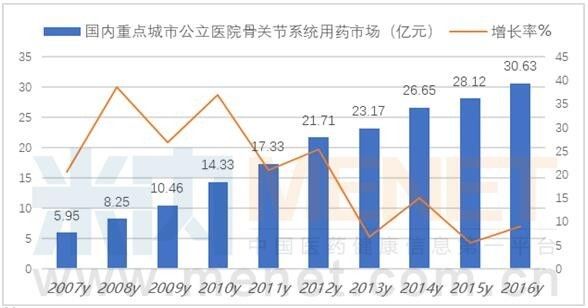

Rheumatism, rheumatoid 48 billion market, targeted drugs
Medical Network October 13th October 12th is World Arthritis Day. According to the World Health Organization, there are 355 million people with arthritis worldwide. With the advent of an aging society, there are more than 100 million arthritis patients in China under the influence of obesity, diabetes epidemics and environmental factors.
Arthritic diseases are composed of two different diseases, rheumatism and rheumatoid arthritis, and the two cannot be confused. Rheumatoid arthritis is a common acute or chronic connective tissue inflammation; rheumatoid arthritis is an immune disease that affects the body. Chinese rheumatoid arthritis accounts for about 0.4%. There are more than 4 million rheumatoid arthritis patients in the country, accounting for 16.88% of the global rheumatoid arthritis group. The number of female patients is three times that of males . Middle-aged women are more likely to develop diseases. However, there is a possibility of morbidity at any age, and the remission rate of Chinese rheumatoid arthritis is only 8.6%, and the disability rate is as high as 50.3%, which seriously affects the lives of individuals and families.
Osteoarthritis market rigid demand
Osteoarthritis is a common and frequently-occurring disease, and it is also a degenerative disease. It is a local lesion of bone and joint caused by aging and rheumatism. Knee ligament injury, meniscus injury, intra-articular fracture or knee surgery have a higher probability of osteoarthritis, and the age of onset is also advanced; congenital knee varus or knee valgus also causes uneven knee weight. It is also a high-risk group of osteoarthrosis. Mainly using joint replacement and medical treatment methods. With the advent of new drugs, chemical drugs, bioengineered antibodies, and proprietary Chinese medicines and peptide-based adjuvant therapies have gradually attracted human attention.
Market category structure change
At present, the treatment of rheumatoid arthritis and rheumatoid arthritis mainly consists of five major categories: non-steroidal anti-inflammatory drugs, anti-rheumatic drugs, biological antibodies, glucocorticoids, and proprietary Chinese medicines.
In 2016, the overall market for rheumatoid arthritis and rheumatoid arthritis in the world's top 500 drugs reached 52 billion yuan, up 6.99% year-on-year. The leading TOP10 varieties of sales are adalimumab, infliximab, etanercept, utekalbab, golimumab, abatacept, susumizumab, tobuzumab, cytoto Zumuzumab and Apster. Antibody drugs account for 10 seats, accounting for 90% of the global market for rheumatoid arthritis and rheumatoid arthritis.
The domestic market is very different
China started late in rheumatology and rheumatoid arthritis, but it has developed rapidly. The Chinese Medical Association Rheumatology Branch "Guidelines for the Diagnosis and Treatment of Rheumatology" and "Guidelines for the Diagnosis and Treatment of Rheumatoid Arthritis" have refined and standardized the application of this class of drugs. Not only has the doctor and patient benefited, but it has also boosted the market for rheumatoid and rheumatoid arthritis.
According to the data from the intranet, the market for bone and joint medication in public hospitals in key cities in China has reached 3.063 billion yuan in 2016, an increase of 8.91% over the previous year, including anti-inflammatory, anti-rheumatic drugs, topical muscles and peptides. 66 varieties such as drugs. According to the "2017 Blue Book of China's Pharmaceutical Market Development", the domestic rheumatism and rheumatoid arthritis drug market is 48 billion yuan, of which chemical drugs account for 66.31%, proprietary Chinese medicines account for 24.60%, and biological products account for 9%. Throughout the domestic clinical drug market, basic drugs are gradually in line with foreign countries, and there is still a big gap in the high-end market.
Domestic bone joint TOP10 drug
According to the data from the intranet, in 2016, the TOP10 drugs for bone joints in public hospitals in key cities in China accounted for 78.26% of the total market. Specific varieties are flurbiprofen ester, glucosamine, deer melon polypeptide, recombinant human type II tumor necrosis factor receptor-antibody fusion protein, parecoxib, bone peptide, hydroxychloroquine, bone melon extract, sodium hyaluronate and Celecoxib.
It can be seen from the above varieties that the best-selling antibody drugs in foreign countries have not yet entered the domestic market. Due to its high price and lack of access to health insurance, the vast majority of patients have no access to the high-end drug market. The only domestic bioengineered drug for rheumatoid arthritis is the recombinant human type II tumor necrosis factor receptor-antibody fusion protein for injection. Overall, the probability of using bioengineering drugs is still far from that of foreign countries.
Targeting agent flurbiprofen leads
Flurbiprofen is one of the excellent varieties of non-steroidal anti-inflammatory analgesics, and it is also a drug with strong action and small therapeutic dose in similar drugs. For the treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, traumatic pain and other pains.
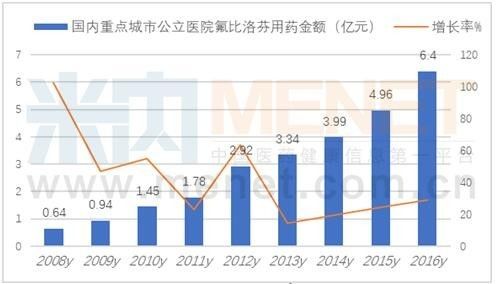
According to the data of the intranet, the amount of flurbiprofen used in public hospitals in key cities in 2016 was 640 million yuan, an increase of 28.88% over the previous year. Beijing Tide Pharmaceutical's flurbiprofen ester injection "Kay" occupied 83.59%, flurbiprofen Babu cream "Zepusi" accounted for 16.41%, the domestic flurbiprofen market has more than 4 billion scale.
Flurbiprofen ester targeted injection "Kay" is a non-steroidal analgesic with lipid microspheres as a drug carrier. The drug enters the body and is distributed to the joint lesions to be released from the lipid microspheres, inhibiting the synthesis of prostaglandins and exerting an analgesic effect. Kay is well tolerated and can accurately achieve the role of the lesion site, to achieve the purpose of anti-inflammatory and analgesia of bone joints. Zepus is manufactured by Japan Sanken Co., Ltd. and is packaged by Beijing Tide Pharmaceutical Co., Ltd. It is a topical patch with strong transdermal strength, fast onset and remarkable analgesic effect.
Recombinant human type II tumor necrosis factor receptor- antibody fusion protein bright spot
After successful use of biological agents for the treatment of rheumatism, it indicates that human resistance to rheumatism has gradually entered the biomolecular drug space. TNF-α antagonist is the most widely used biological preparation. The recombinant human type II tumor necrosis factor receptor-antibody fusion protein developed in China has become a bright spot in TNF-α antagonists.
Bioengineered preparations are significantly superior to non-steroidal anti-inflammatory drugs for rheumatoid diseases and play a role in the treatment of rheumatoid arthritis, ankylosing spondylitis and adult moderate and severe plaque psoriasis. The original drug etanercept was introduced to the Chinese market in 2012, and the excessive price has affected the further expansion of its market.
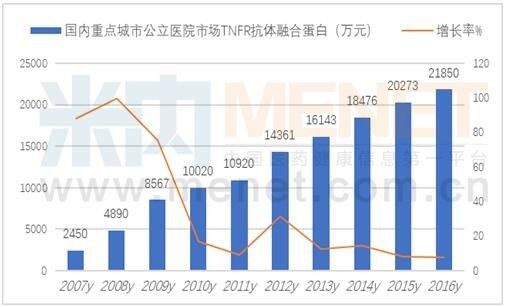
In 2005, Sansheng Guojian Pharmaceutical (Shanghai) was the first to obtain a recombinant human type II tumor necrosis factor receptor (TNFR)-antibody fusion protein registration approval, the trade name is Yisaipu. Subsequently, in 2011, Shanghai Saijin Biomedical TNFR antibody fusion protein was approved, and the trade name was Qianke; in 2015, Zhejiang Haizheng Pharmaceutical's similar drugs were approved for listing, and the trade name was Anzhenuo; thus forming a three-legged pattern.
According to the data from the intranet, the market for TNFR antibody fusion protein in public hospitals in key cities in 2016 was 219 million yuan, an increase of 7.78% over the previous year. Statistics show that the domestic recombinant human type II tumor necrosis factor receptor-antibody fusion egg clinical drug has reached a market scale of 1.68 billion yuan. Yishengpu of Sansheng Guojian Pharmaceutical (Shanghai) occupies 60% of the TNF-α antagonist market. The 2017 edition of the National Medical Insurance Drug List contains recombinant human type II tumor necrosis factor receptor-antibody fusion protein, which is of great significance for future market volume.
New domestic bone and joint products come one after another
Tofacitinib is Pfizer's first novel oral targeting drug, JAK kinase inhibitor, for rheumatoid arthritis. Clinically used in the treatment of rheumatoid arthritis can block the signaling of inflammatory cytokines in the cell that can cause rheumatoid arthritis. It is the only JAK kinase inhibitor that has been included in the American College of Rheumatology 2015 Guidelines for the Treatment of Rheumatoid Arthritis. It has been approved for marketing in more than 80 countries around the world. According to Pfizer's annual report data, the global Tofa-Tib market in 2016 was 927 million US dollars, with a growth rate of 77.25%.
On March 10, 2017, China CFDA approved the registration of Pfizer to produce tofatib, the trade name of Xeljanz. Officially launched in China on August 13, 2017; for the treatment of adult patients with moderate to severe active rheumatoid arthritis (RA).
According to the public information of the CFDA official website, China Resources Secco Pharmaceuticals (CXHL1400029/30 Beijing) and Qilu Pharmaceutical (CXHL1400764/65 Lu) have developed and applied for the raw materials and tablets of tofatib. It is said that Haizheng Pharmaceutical has obtained the approval of the Fabribu (2016L03854). So far, more than 30 pharmaceutical companies in China have been involved in the development of tofatib. (Cai Deshan)