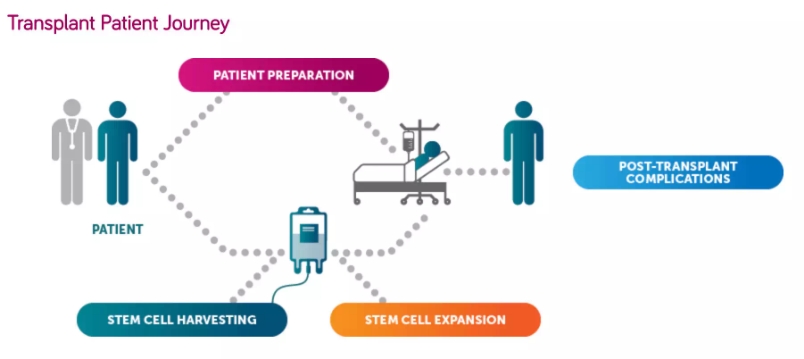
Cord blood stem cell therapy is coming? The first patient has been treated April 09, 2018 Source: WuXi PharmaTech Recently, Magenta Therapeutics announced that in the Phase 2 clinical study of MGTA-456 cord blood stem cell transplantation, the first patient with abnormal genetic metabolism was treated. Genetic metabolic disorders can lead to disability and premature death in affected children. Bone marrow transplantation is often the preferred or only available treatment. However, many patients often do not have well-matched stem cell donors. Cord blood can provide a good source of stem cell matching for these patients because cord blood transplantation requires less human leukocyte antigen (HLA) binding sites. Studies have shown that bone marrow transplantation needs to meet 100% matching of a total of 6 HLA sites, while cord blood transplantation only needs to meet 67% matching of 4 sites, with fewer side effects, viral infection and severe graft versus host disease. (GVHD) has a low incidence. However, cord blood transplantation is usually limited by the typical low stem cell dose of cord blood, especially in adult cord blood transplants. Magenta Therapeutics is a biotechnology company dedicated to developing new drugs to provide bone marrow transplant treatment to more patients. It is currently developing methods that allow stem cells to expand and maintain cell migration capabilities, delivering optimal numbers of healthy stem cells to patients and improving stem cell therapy outcomes. Magenta Therapeutics' most advanced candidate, MGTA-456, is a "first-in-class" allogeneic stem cell therapy consisting of a single cord blood unit and an aromatic receptor (AHR) antagonist that can pass through the bone marrow. The transplant is administered to the patient. AHR antagonism is a novel, clinically proven pathway to control cell self-renewal and differentiation. Previous clinical studies have shown that MGTA-456 is the only hematopoietic stem cell (HSC) graft that produces fast, robust implants. The rapid implantation of all patients demonstrates that the expansion process leads to a significant expansion of HSCs – a long-term goal in this area. Through this expansion process, MGTA-456 has the potential to allow patients to receive higher cell dose treatments and obtain better HLA-matched cord blood units, both of which have been shown to provide better results and lower transplant concurrency The incidence of disease, which was not possible in the past. ▲Bone marrow transplantation process (Source: Magenta Therapeutics official website) MGTA-456's Phase 2 clinical trial plans to recruit 12 patients with Hurler's syndrome (alpha-L-iduronidase gene deficiency), adrenal leukodystrophy, metachromatic leukodystrophy or spheroid cell brain White matter malnourished patients. The primary endpoint was post-transplantation and the secondary endpoint was graft-related safety and tolerability. The study is currently enrolling patients at the University of Minnesota and may be extended to other locations in the future. "MGTA-456 has demonstrated clinical evidence of its concept in 36 patients with hematological cancer, and we are now exploring its potential in patients with hereditary metabolic diseases," said Dr. John Davis, Chief Medical Officer of Magenta Therapeutics: "Because of MGTA -456 is able to significantly amplify a single cord blood unit to a higher cell dose, which increases the likelihood and speed of implantation, which we believe will be a powerful therapeutic option for these patients. We intend to be in other debilitating diseases In the study of MGTA-456, we believe it can bring transformative benefits to patients." Magenta Therapeutics pioneered a comprehensive approach to study pre-transplant preparation, stem cell harvesting, stem cell expansion, and post-transplant complications. We look forward to bringing revolutionary bone marrow transplants to patients with autoimmune diseases, blood cancers and genetic diseases by making the process more effective, safe and easy. Reference materials: [1] Magenta Therapeutics Announces First Patient Transplanted with MGTA-456 in Phase 2 Study in Inherited Metabolic Disorders [2] Magenta Therapeutics official website [3] Magenta Therapeutics' Expanded Stem Cell Product, MGTA-456, Engrafts in 100% of Patients, Leading to Robust Neutrophil and Immune Recovery in Two Phase 2 Clinical Studies
Disposable latex gloves are divided into the following three levels. One is the powdered disposable latex gloves that are mostly used in the food industry. It is necessary to add them in the production process to prevent the gloves from sticking together and to facilitate wearing. It is important to note that there are good and bad cornmeal. We use food grade corn flour, otherwise it is not good for the user and the person being served. The second is the powder-free disposable latex gloves that are mostly used in the electronics and medical industries, because they are powdered when they are just produced, and the powder-free latex gloves obtained after our processing - washing with water. Third, they are mostly used in the precision electronics and medical industries. The purifying disposable latex gloves are made of powder-free latex gloves that have been washed with clean water and washed with chlorine again, and the cleanliness reaches a thousand grades.
Latex Gloves,Rubber Gloves,Rubber Hand Gloves,Pink Latex Gloves Jiangsu Asbao Medical Technology Co., Ltd. , https://www.iigloves.com