Chinese team breaks through the bottleneck of CAR-T therapy and is expected to change the treatment pattern April 24, 2019 Source: Academic Jingwei On April 23, "Nature Medicine" published in Nature, an important medical research led by Professor Zhu Jun of Peking University Cancer Hospital and Professor Chen Siyi of the University of Southern California - scientists have found the star anti-cancer therapy CAR -T became a safer method and was validated in early human clinical trials. Many experts pointed out that this discovery is expected to change the treatment pattern of CAR-T. CAR-T is a star anti-cancer therapy that has emerged in recent years. This therapy separates immune T cells from patients and genetically modifies these cells in vitro, and attaches them to a "chimeric antigen receptor" (CAR) that recognizes cancer cell surface antigens. Subsequently, these engineered cells are extensively expanded in the laboratory and infused back into the patient. There, they are like a well-trained army equipped with the latest weapons, and ruthless attacks on cancer cells. The concept and efficacy of this therapy has been validated in a series of clinical trials. In 2017, the US FDA approved the launch of the first CAR-T therapy. A few months later, the second CAR-T therapy was approved. Some children who have received this therapy since clinical trials have not experienced cancer recurrence in 5 or 6 years, achieving a "functional cure." But researchers know that CAR-T therapy is not a panacea. Because these immune T cells are regarded as a "drug", they often cause side effects of "cytokine storm" in the human body, and sometimes even endanger the patient's life. Therefore, current patients must be treated with CAR-T in a well-equipped, medical institution with specialized knowledge to control potential side effects in a timely manner. To understand whether it is possible to further improve the safety of CAR-T therapy and reduce the birth of cytokine storms, the researchers decided to modify the "chimeric antigen receptor" in the first approved CAR-T therapy. It can maintain efficacy, enhance safety and reduce the release of cytokines. After extensive screening, a chimeric antigen receptor called CD19-BBz (86) has attracted the attention of researchers. In mouse experiments, it effectively kills cancer cells without causing serious immune side effects. This is exactly what they are looking for. Next, they applied for a clinical trial to investigate whether this improved CAR-T therapy can lead to safer treatments in human patients. This early clinical trial enrolled 26 patients with refractory B-cell lymphoma, 25 of whom were treated. Similar to previous results, CAR-T therapy has achieved excellent results in combating cancer: in both low- and medium-dose groups, half of patients have clinical remission. Of the 11 patients who received the highest dose, 6 achieved complete remission (54.5%) and 2 achieved partial remission (18%), indicating that the improved CAR-T therapy was not compromised. And the new CAR-T therapy can be said to be a big win in the safety that researchers are most concerned about. None of the 25 patients had a cytokine storm of level 1 or higher. Even more gratifying is that no patient has neurotoxicity (another common side effect of CAR-T therapy). Taken together, no patient needs medication to alleviate side effects. Compared to the existing approved CAR-T therapy (more than half of which are plagued by cytokine storms, about a quarter of which are neurotoxic), the positive results of the research are exciting! “This is an important step forward,†said Professor Chen Siyi, one of the heads of the study. “We designed a new type of CAR molecule that is as effective in killing cancer cells, but at a less intense rate and toxicity. Lower." Once published, this study has also caused heated discussion among many industry experts. Some experts point out that although this study only recruited 25 patients, early data has great potential. Other experts expressed cautious optimism. Although we have achieved the desired results, why this design can reduce toxicity, the mechanism behind it remains to be further explored. In summary, the findings made by the team led by this Chinese scholar are expected to lead to safer CAR-T therapy. If this can be verified in more clinical trials, it is a boon to the patient. After all, safer therapies will not only make their treatment more reassuring, but also prevent the weak patients from going to the special medical center to get the treatment. This is exactly what biomedicine is actually making for patients. Xi'an Healthway Biotech Co.,Ltd , https://www.xianhealthway.com
â–² A large number of Chinese scholars participated in this study (Source: Screenshot of Nature Medicine) 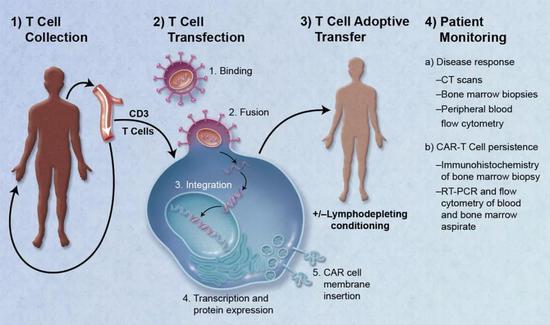
â–² CAR-T therapy is a major breakthrough in cancer in recent years (Source: Caron A. Jacobson and Jerome Ritz [Public domain]) 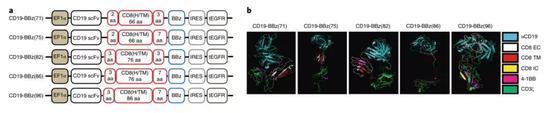
â–² Researchers have different designs for chimeric antigen receptors 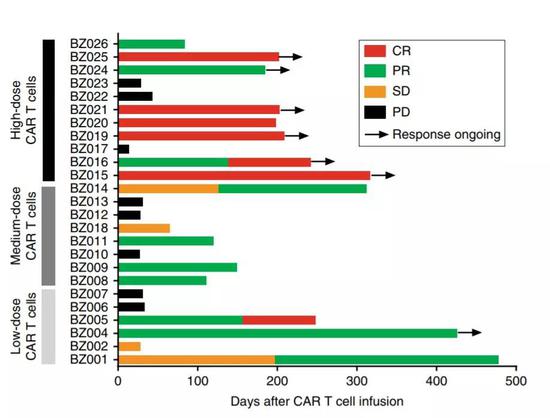
â–²The effect of this therapy is still outstanding