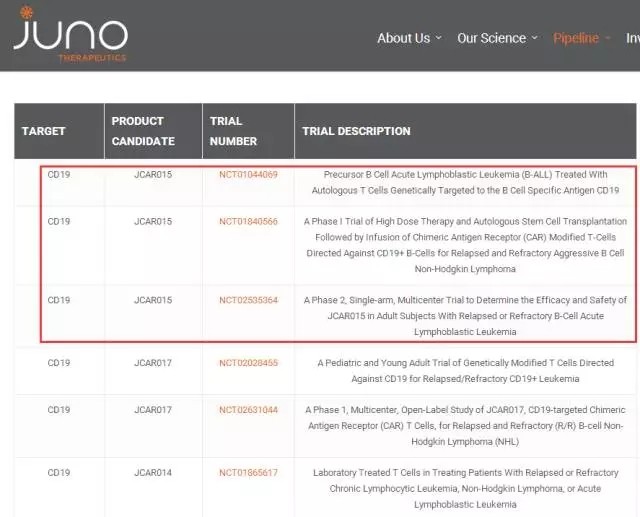
On January 22, Celgene and Juno Therapeutics announced the signing of a merger agreement. According to the terms of the merger agreement, Celgene will pay Juno cash of $87 per share for a total of approximately $9 billion in cash and securities, Celgene It will receive approximately 90% of the outstanding shares of Juno's shares. The transaction has been approved by the board of directors of both companies. Just this month, Celgene has just signed an agreement to acquire Impact Biomedicines, according to which Celgene will pay $1.25 billion based on the FDA approval milestone for the myelofibrosis project. Payments for future sales, etc. can be as high as $4.5 billion. So the total value is expected to reach $7 billion. For the acquisition of Juno by Celgene, Celgene will become another giant company in the "competition field" of CAR-T, which is exposed to Juno's CAR-T star blood cancer drug JCAR017. Last summer, Gilead Sciences purchased Kite Pharma for an amazing $12 billion in funding for its CAR-T therapy. CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy), a chimeric antigen receptor T cell immunotherapy, is a target gene that recognizes tumor cells by genetic engineering by separating T cells from patients. It can specifically recognize cancer cells, and then inject them into the patient after being expanded in vitro to achieve the effect of eliminating cancer cells. At present, CAR-T therapy shows a high rate of remission in acute lymphoblastic leukemia and non-Hodgkin's lymphoma. Juno production line review Juno's CD19-targeted CAR-T cell therapy products are available in three categories: JCAR015, JCAR017, and JCAR014. JCAR015 is a second-generation CAR-T cell product (hereinafter referred to as CD28zCAR) with CD28 as a costimulatory domain. The viral vector used is a retrovirus for the treatment of adult relapsed refractory acute B-lymphocytic leukemia (B-ALL) and Non-Hodgkin's lymphoma (NHL). Phase I clinical trials have been conducted for acute B-lymphocytic leukemia (B-ALL) indications, and phase II clinical trials have been suspended. JCAR017 is a second-generation CAR-T cell product (hereinafter referred to as 4-1BBzCAR) with 4-1BB as a costimulatory domain. The viral vector used is a lentivirus for the treatment of relapsed and refractory acute B-lymphocytic leukemia in children and adults. (B-ALL) and non-Hodgkin's lymphoma (NHL). Phase I/II clinical trials are being conducted for acute B-lymphocytic leukemia (B-ALL) indications, and Phase I clinical trials are being conducted for non-Hodgkin's lymphoma (NHL) indications. According to reports, the complete response rate of JCAR017 was 91%. JCAR014 is also a second-generation CAR-T cell product with 4-1BB as a costimulatory domain. The viral vector used is also a lentivirus. Unlike JCAR017, JCAR014 uses a mixture of CD4+ and CD8+ (1:1). Cell population. Indications include relapsed refractory adult chronic B lymphocytic leukemia (B-CLL), acute B lymphocytic leukemia (B-ALL), and non-Hodgkin's lymphoma (NHL). I/II clinical trials are currently underway. According to reports, the complete response rate of JCAR014 for B-ALL indications is 100%. In July 2016, five patients with acute lymphocytic leukemia (ALL) died after receiving a clinical trial of Juno named ROCKET. This project called ROCKET was used for Juno CAR-T therapy. The main product, the clinical test of JCAR015, involved 38 ALL patients. In November 2016, JUNO's CAR-T therapy JCAR015 codenamed ROCKET (Rocket) Phase II study, two more patients with cerebral edema, one of them has died, and the other one is "not expected to recover" JUNO took the initiative to suspend, and in March the following year officially announced the abandonment of the clinical research of CAR-T product JCAR015. In December 2016, Juno's JCAR017 received FDA breakthrough drug certification for refractory, recurrent advanced non-Hodgkin's lymphoma, including diffuse large B lymphoma, primary mediastinal B-cell lymphoma, and other types of lymph Clinical treatment of tumors. On November 1, 2017, Juno Therapeutics announced that the CD19-targeted CAR-T cell drug, JCAR017, was included in the core analysis group of 49 patients (n = 49), including representatives of Juno's ongoing key cohort study. patient. The overall response rate for 3 months was 80% (n=12/15) and the complete response rate was 73% (n=11/15). It is reported that Juno is expected to apply to FDA for JCAR017 this year and is expected to obtain listing approval in 2019. Celgene sees the drug as "an important growth driver after 2020," with potential sales of $3 billion. At the same time, Celgene will have to fight with Novartis and Gilead, both of which have considerable lead in the market. Celgene CEO Mark J. Alles Celgene CEO Mark J. Alles said: "The acquisition of Juno builds on our shared vision to discover and develop translational drugs for incurable blood cancer patients. Juno's advanced cellular immunotherapeutic portfolio and research capabilities reinforce Celgene's global leadership in hematology, Adding new impetus to growth beyond 2020." Juno President and CEO Hans Bishop also said: "Juno's R&D staff is passionate about science and patients. Our common goal is to find cell therapy that can cure diseases and help people live longer and live better. Continuing this work requires scientific power, superior production capacity and a global network layout. This merger provides us with the above three points." Future strategic deployment After the acquisition of Juno, Celgene will deploy the following three aspects: 1. Create innovative research platforms and scalable manufacturing capabilities to guide Celgene's future position in cellular immunotherapy science. 2. Accelerate Juno's pipeline development and maximize the potential of cellular immunotherapy. JCAR017 is used for the marketing of lymphoma treatment and the development of JCARH125 for the treatment of key drugs for multiple myeloma. Adding cell therapy assets to Celgene's existing line for proof-of-concept trials for hematologic malignancies and solid tumors. 3. After 2020, the growth of meaningful research projects for the treatment of refractory cancer through the CAR-T research field will drive income diversification. Lake Smelt,Lake Superior Smelt,Pond Smelt,Pond Smelt Fish ZHEJIANG EVERNEW SEAFOOD CO.,LTD , https://www.evernewseafood.com